本帖最后由 Aiden6 于 15-10-2020 17:12 编辑
Purifa company supplies Buda-U FFP2 face Mask. If you need them, please contact us.
Price negotiable.
The general situation of FFP2 face Mask manufacturing company is as follows:
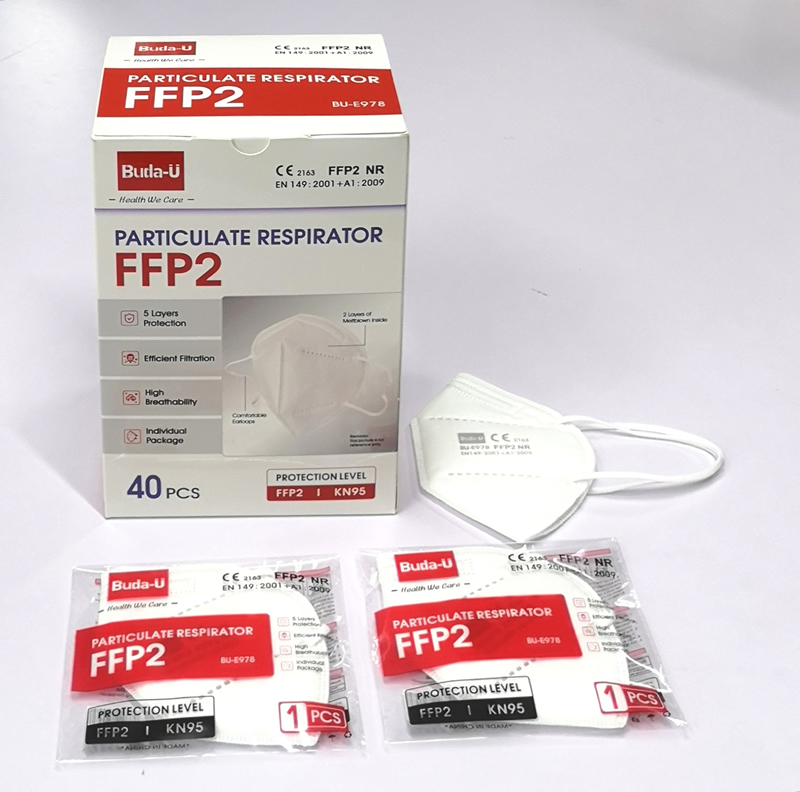
Key Specifications/Special Features:· FDA EUA Authorization, FDA Certification & Registration. · CE (Module B+C2 , NB2163) , FFP2 Grade; · Meeting All Requirements of EU Standard : EN 149:2001 + A1:2009, FFP2 ; · KN95 Approved, China Standard: GB2626-2019; · ≥95% Filtration efficiency against Non-Oil Particulates; · 5 Layers in which 2 Layers are Meltblown; · Individual Package for Every Single Piece; · Firm, Elastic & Comfortable Earloops Fitting Different Face Shape · Packing Box and Individual package with Different Global EAN Barcodes, · MFG and EXP. Date Actively Printed on Packing Box ; · 40 Pcs / Box , 800 Pcs / Carton .
Buda-U Masks Spread Over the World
Buda-U trademark has been registered not only in China, but also in the U.S. and EU in order to echo its global marketing expansion. There has been a significant increase of sales in the EU and U.S. over recent months. Buda-U's products can be easily seen in the market in many countries. And we are establishing distribution channels with our buyers across the world.

About Buda-U
Buda-U is the professional producer committed to producing Personal Protective Equipment (PPE) such as FFP2 Filtering Half Masks, KN95 Particulate Respirators and 3PLY Disposable Face Masks for adults and children. Buda-U is the brand of PURIFA Medical and It is the mask exporter and manufacturer in China White List. Buda-U KN95 has been authorized by U.S.A FDA as EUA product.
Buda-U’s main production base, in Dongguan, China, produces over 200,000 pieces of FFP2/KN95 masks and over 150,000 pieces of 3-ply disposable masks per day. Our dust-free workshops run 24 hours a day in order to meet the increasing demand of protective products for our customers across the globe.
Buda-U Certifications and Qualifications · FDA EUA Authorized, FDA Device Listed and Registration. · In China CCCMHPIE White List. · Buda-U KN95 Masks have got the CE certification of Module B+C2 by EU Notified Body No.2163, with the classification of FFP2 grade under EU Standard of EN 149:2001 +A1:2009. · 3PLY Disposable Medical Masks have conducted the Attestation of Conformity and meet all the requirements of the EU medical standard of EN14683: 2019 and are in conformity with the provisions of MDD /MDR 93/42/EEC.
We are doing everything that we can to ensure good product quality. Buda-U team is committed to consistently achieving customer satisfaction by delivering best-value products through efficient practice of internal quality management. Buda-U's mission is to consistently provide reliable products that can deliver value to customers in health care and protection.
Contact Buda-U:
Contact No.: +8613929231627 Ms. FIONA
Email : support_bu@126.com
Wechat ID: Buda-U
Website: Buda-U.com
PURIFA Medical Production Co.,LTD.
Add: No.128, Langchang Rd., DaLang, Dong Guan, Guangdong, P.R. China.
|